
HL Paper 3
A polarimeter can be used to determine the optical rotation of an optically active substance.
Describe what happens to plane-polarized light when it passes through a solution of an optically active compound.
A mixture of enantiomers shows optical rotation.
Suggest a conclusion you can draw from this data.
(i) Identify the two reagents used to form the electrophile in the nitration of benzene.
(ii) Explain, using curly arrows to represent the movement of electrons, the mechanism for this reaction.
Chirality plays an important role in the action of drugs.
Using an asterisk (*), identify the chiral carbon atom in the structure of thalidomide.
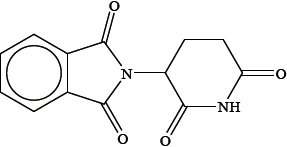
Describe the composition of a racemic mixture.
Identify the organic product in the following reaction.
\[{{\text{C}}_6}{{\text{H}}_6} + {\text{HN}}{{\text{O}}_3}\xrightarrow{{{{\text{H}}_2}{\text{S}}{{\text{O}}_4}}}\]
Vision is dependent on retinol (vitamin A) present in retina cells. Retinol is oxidized to the photosensitive chemical 11-cis-retinal and isomerizes to 11-trans-retinal on absorption of light.
Outline how the formation of 11-trans-retinal results in the generation of nerve signals to the brain.
The action of a drug molecule often depends on its shape. Discuss a specific example of a drug where one stereoisomer has a different physiological activity to the other.
Different conventions are used to classify enantiomers. Orange and lemon peel each contain different enantiomers of the compound limonene. One of the enantiomers is represented below.
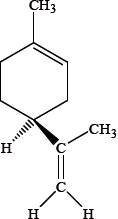
Identify the chiral centre in this enantiomer with an asterisk, *.
The \(( + )d\)-enantiomer has the characteristic smell of oranges and the \(( - )l\)-enantiomer has the characteristic smell of lemons. Explain the meaning of the \(( + )d\) and \(( - )l\) symbols used in this notation.
The R, S notation is also used. The \(( + )d\)-enantiomer is often described as R-limonene and the \(( - )l\)-enantiomer as S-limonene. Explain what is meant by the R, S notation and state whether the structure shown is R or S.
The structure below shows –(l)-carvone.
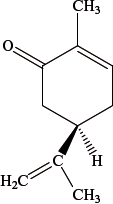
–(l)-carvone has another optical isomer.
State its name and, by means of a diagram, predict its structure.
Describe the structural feature of the carvone molecule that allows it to exist as optical isomers.
Amino acids, shown in section 33 of the data booklet, can be combined to form polypeptides and proteins.
(i) Serine is a chiral amino acid. Draw both enantiomers of serine.
(ii) State the enantiomeric form of serine found in proteins.
Methylbenzene can be prepared from benzene and iodomethane.
Methylbenzene reacts when heated with a mixture of concentrated sulfuric acid and concentrated nitric acid.
(i) Name the major organic products formed.
(ii) Identify the electrophile in this reaction and explain how it is formed.
Amino acids are the building blocks of proteins.
Draw the structures of the main form of glycine in buffer solutions of pH 1.0 and 6.0.
The pKa of glycine is 2.34.
Calculate the pH of a buffer system with a concentration of 1.25 × 10−3 mol dm−3 carbonic acid and 2.50 × 10−2 mol dm−3 sodium hydrogen carbonate. Use section 1 of the data booklet.
pKa (carbonic acid) = 6.36
Sketch the wedge and dash (3-D) representations of alanine enantiomers.
UV-Vis spectroscopy can be used to determine the unknown concentration of a substance in a solution.
Calculate the concentration of an unknown sample of pepsin with an absorbance of 0.725 using section 1 of the data booklet.
Cell length = 1.00 cm
Molar absorptivity (extinction coefficient) of the sample = 49650 dm3 cm−1 mol−1
A different series of pepsin samples is used to develop a calibration curve.
Estimate the concentration of an unknown sample of pepsin with an absorbance of 0.30 from the graph.
In recent years several antiviral medications have been produced. One of these medications is oseltamivir (Tamiflu).
Identify the functional group circled in the structure of oseltamivir.
Predict the number of signals and relative integration you would expect to see in the nuclear magnetic resonance spectroscopy (1H NMR) spectrum for the circled portion in the structure.
Number of signals:
Relative integration:
Oseltamivir is a chiral compound.
(i) Identify an apparatus that can be used to distinguish between its enantiomers.
(ii) Explain how the differentiation between the enantiomers is obtained using this apparatus.